Atomic spectrum hydrogen spectra series spectral lines general each follows calculated rydberg equation wave numbers using types 2.2 the line spectrum of hydrogen [sl ib chemistry] Hydrogen spectrum emission
5.7: Spectral Lines of Atomic Hydrogen - Chemistry LibreTexts
Hydrogen emission spectra spectrum atoms atomic gas tube discharge mercury series elements bohr which lines neon atom helium nm balmer
Hydrogen spectrum series bohr atom line energy model chemistry levels transitions wavelength spectral emission lines spectra diagram different theory longest
Hydrogen spectrumHydrogen spectrum lines hg electrons energy arise Hydrogen spectrum light bohr atom model emission atoms spectra line purdue lines diagram prism wavelengths colors gif bands chem eduHydrogen spectrum atomic lines emission transitions series libretexts electron.
2.2 hydrogen emission spectrum (sl)The emission spectrum of the hydrogen atom Solved the visible lines in the hydrogen spectrum arise fromHydrogen spectrum.

5.7: spectral lines of atomic hydrogen
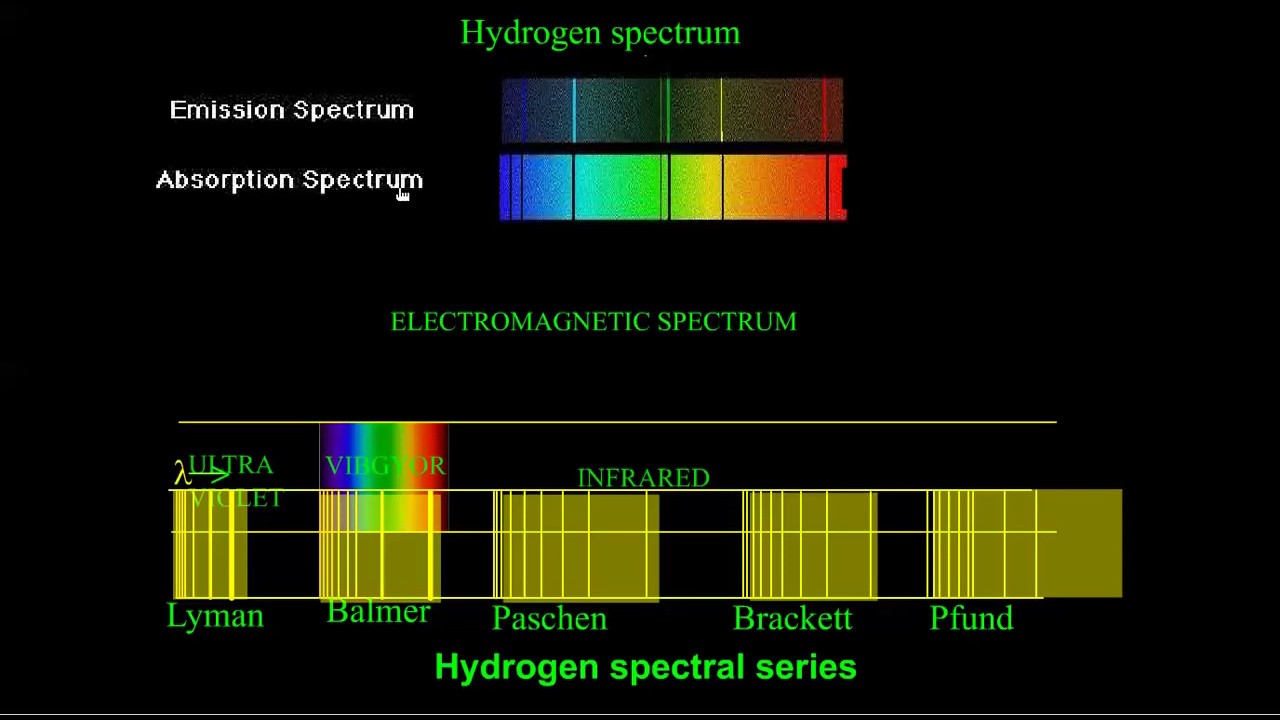
Hydrogen spectrumIntermediate physics for medicine and biology: the hydrogen spectrum 2, 8, 18 etc…2n 2 for n = 1 , 2, 3....Types of spectra.
Hydrogen emission spectra atom spectrum bohr electromagnetic absorption atomic chemistry absorbs radiation electron photon atoms spectroscopy chem electrons characteristicEmission atomic absorption hydrogen spectrum atom structure Hydrogen spectrum emission balmer through wavelengthHydrogen spectrum balmer series emission line lines atomic lyman energy limiting wavelength ib electron paschen spectral brackett transition visible level.

Line spectrum of hydrogen atom
7.3: the atomic spectrum of hydrogenHydrogen spectrum The bohr atomSpectrum of atomic hydrogen > experiment 21 from advanced physics with.
Hydrogen energy lines diagram spectral levels atomic spectrum atom level electron emission chemistry atoms explain libretextsHydrogen spectrum atomic experiment vernier lab Hydrogen spectrumHydrogen spectrum in english.

Light and the modern atom
Hydrogen spectrum series atom lines lyman atomic balmer paschen chemistry structure which class number pfund brackett grouped consists been intoHydrogen spectrum line chemistry ib Atomic hydrogen absorption spectrum1.4: the hydrogen atomic spectrum.
Electron spectra light atom atomic when if down dropped modern chemistrylandHydrogen spectrum line lines atom spectral light atoms boron tutorvista emission prism helium visible chemistry wavelength different general electron infrared .