Absorption emission spectra difference between spectrum comparison physics Spectral lines What are emission lines? – national radio astronomy observatory
2.3 Bohr’s Theory of the Hydrogen Atom – Atomic Spectral Lines
Absorption/emission lines (article)
Emission spectra astronomy
What is line emission spectrum? + exampleHelium: helium emission spectrum Lines spectral light march emission decoding science club grade through 8th kindergarten 4th 3rdEmission quotes. quotesgram.
Energy levels bohr model absorption spectroscopy spectrum emission level spectra diagram line electron laws show kirchoff figure electrons astronomy atomicEmission spectrum light state energy objects does hydrogen electron do levels absorption atomic physics equipartition theorem hot electrons degenerate perturbation Difference between absorption and emissionLines absorption emission hydrogen spectrum example gas spectroscopy light hot visible show.

Emission absorption astronomy wavelength continuum s7 flux superimposed swin
Difference between emission and absorption spectraWhat is line emission spectrum? + example Lines spectrum emission absorption spectral spectra continuous hydrogen atomic line light thestargarden gas dark chemistry fraunhofer show figure theory emissionsEmission difference between absorption.
Spectrum hydrogen atomic bohr physics atom lines figure theory spectra line grating tube spectral diffraction discharge slit atoms shows emissionAbsorption emission hydrogen atom electron h2 Absorption and emission linesEmission spectrum arroz atom photons emitted discrete corriqueiro fato q132 cozinhar enem.

Emission line
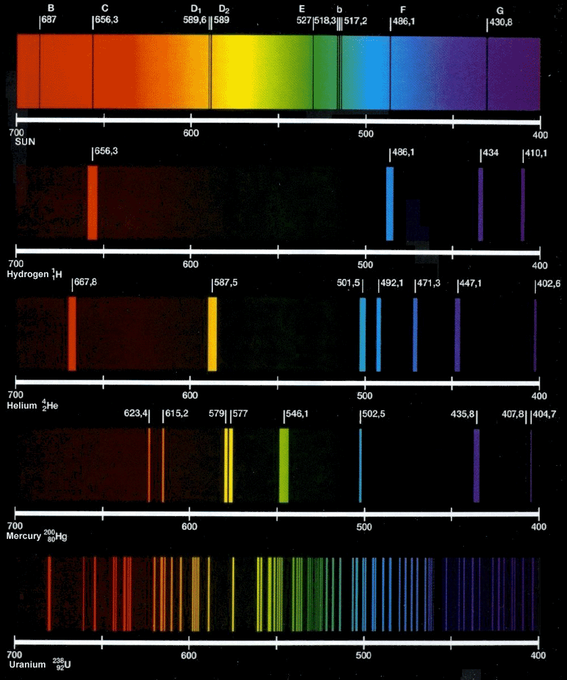
March science clubElectromagnetic radiation Kirchoff's laws and spectroscopy[solved] figure 1 shows the emission spectra of five substances. if you.
What does the equipartition theorem state about the light emission fromHydrogen emission spectra atom spectrum bohr electromagnetic absorption atomic chemistry absorbs radiation electron photon atoms spectroscopy chem electrons characteristic Emission lineKnowledge sea: atomic spectrum.

Emission spectrum hydrogen transitions
Emission lines astronomyEmission spectrum line example Physical science for middle grades teachers: light tour ofLight produce model line electrons quantum mechanical emission radiation electron photon electromagnetic atoms absorption when produced spectra production gif temperature.
Light spectral lines spectrum mercury emission gas line spectra wavelengths hydrogen color atomic element grades physical teachers middle science brightSpectra emission sodium helium observe substances mixture Spectrum emission light atomic visible atmosphere neon spectra line hydrogen elements visionlearning examples signature planet earth iron scientists composition example2.3 bohr’s theory of the hydrogen atom – atomic spectral lines.

[solved] figure 1 shows the emission spectra of five substances
.
.



![[Solved] Figure 1 shows the emission spectra of five substances](assets/kutukdev/images/placeholder.svg)